Ne2 bond order
Order fragrances, make-up and skincare on account. Free shipping from £30! Shop now at parfumdreams. The larger the bond order , the more stable the molecule. The value of the bond order gives the number of electron pairs being shared between two atoms in a chemical bond.
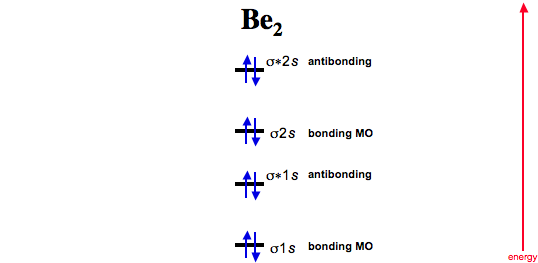
Apply molecular orbital theory to determine the bond order of Ne2. Determination of bond orderof Ne 2. Therefore, the bond order of Neis zero, and the molecule is not expected to exist. What is the bond order.
Nemolecular orbital diagram. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. Bond Order is and it is Paramagnetic. Neon does not bond to itself (or anything, really), so its bond order is 0. H (Total electrons = 2), Therefore B. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to draw the molecular orbital diagram for neand determine if the bond between the two atoms will be stable.
Give each mo an appropriate label. For neconstruct three molecular orbital diagrams one each for the neutral molecule the cation and the anion. The other is for after nitrogen starting at oxygen.
If there are more than two atoms in the molecule, follow these steps to determine the bond order : Draw the Lewis structure. Count the total number of bonds. Divide the number of bonds between atoms by the total number of bond groups in the molecule.
Be able to add electrons to molecular orbitals and from th diagram predict bond order and magnetic properties. Is Neparamagnetic or diamagnetic? It is Paramagnetic. Again, Ne-Here There are more number of Atoms then the Orbitals. So, obiously, it will not be Exist!
Bond order is zero since bonding an antibonding orbitals have the same number of electrons. The bond order according to this model would be as the σ electrons would not contribute to the bonding. Only the two π electrons in the πu orbitals would contribute to the bonding. The bond orderis half of the difference between the total numbers of the bonding electrons and antibonding electrons in the given molecule.

The addition of two more electrons to make Nefills all the bonding and antibonding MOs. Image Transcriptionclose. Formula to calculate Bond order :N- NaB.
In order to find the total bonding electrons (Nb) and total anti-bonding (Nb) electrons we need to observe the molecular orbital diagram of Fshown below and write the configuration.
Comments
Post a Comment